Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Cajal Bodies in Developing Histaminergic Neurons of the Rat Brain
*Corresponding author:Ekaterina M Phedina, Department of Histology, Cytology and Embryology, Grodno State Medical University, Grodno, Bolshaja Troickaja str, 4, 230023, Republic of Belarus
Received:January 23, 2023; Published:February 01, 2023
DOI: 10.34297/AJBSR.2023.17.002420
Abstract
Objective: The aim of the study is to assess the features of Cajal bodies formation in rat brain histaminergic neurons from the 5th to the 45th days of postnatal ontogenesis.
Material and methods: The scientific work was performed on the offspring of outbred white rats (12 animals). The decapitation of rats was carried out on the 5th, 20th and 45th days after birth. We used histological, electron microscopic, morphometric, and statistical research methods in the work. The obtained data were processed by nonparametric statistics.
Results: From the 5th to the 45th days of the postnatal period, in the nuclei of rat hypothalamus histaminergic neurons, Cajal bodies, mainly in contact with the nucleolus or lying freely, are detected. In the process of establishing close association with the nucleolus, these structures are attached to it, forming a “cap” on its surface. On the 20th day, an increase in the degree of nucleoli association with Cajal bodies is revealed, which is manifested by the formation of perinucleolar ring clusters from Cajal bodies in the form of rosettes.
Conclusion: During the described period of postnatal ontogenesis of histaminergic neurons, Cajal bodies do not undergo significant changes in shape and size, which could be related to the age of the animals. The frequency of occurrence of these structures decreases by almost 2.6 times.
Keywords: Cajal bodies, Histaminergic neurons, Postnatal ontogenesis
Abbreviations:CBs: Cajal Bodies;LQ: Upper Limit of the Lower Quartile; Me: Median; UQ: Lower Limit of the Upper Quartile.
Introduction
Cajal bodies (CBs) are distinct nuclear structures ubiquitous in plant and animal cells [1]. They are first discovered by Santiago Ramón y Cajal in 1903 in the nucleus of a neuron. They have been described as round argyrophilic formations and called “accessory bodies of the nucleolus” because of their often-close proximity to it [2]. In 1969, using electron microscopy, Monneron and Bernhard rediscovered these nuclear inclusions in hepatocytes as coils of electron-dense disordered helical filaments and, based on the revealed structural configuration, called them “coiled bodies” [3]. In the same year, Hardin, and co-workers (1969), reported a similar structure by electron microscopy in sensory ganglion neurons, but retained the name of “accessory body “, because they knew Cajal’s work on the neuronal nucleus [4]. After the publication of these studies results, CBs led a parallel life as a “coiled body” and “accessory body” for more than a decade, until Lafarga and colleagues definitively established that they all have the same structure [5]. In 1999, coiled bodies were renamed “Cajal bodies” in honor of their discoverer [6-8].
One cell can contain from 1 to 10 CBs [9]. Their biogenesis exhibits the properties of a self-organizing structure [10]. There is a close physical relationship between the nucleolus and the CBs. It is especially pronounced in most types of rat neurons [11]. It is known that the Cajal body includes small nuclear and nucleolar ribonucleoproteins involved in pre-mRNA splicing and pre-rRNA maturation [8,12-15], and contains a specific marker - p80-coilin protein and survival motor neuron protein, which play an important role in maintaining the structural integrity of this formation [16]. In addition, CB shares fibrillarin, Nopp140, NAP57, and DNA topoisomerase I proteins with the nucleolus [8,12,17,18], which leads to a functional dialogue between them. Currently, CB is interpreted as a multifunctional structure involved in the biogenesis, transport, and recycling of small nuclear and nucleolar RNAs [16].
In adult mammals and humans, the bodies of histaminergic neurons are in the tuberomammillary region of the hypothalamus, where they form five clusters - nuclei (E1-E5), which are spatially interconnected and gradually pass one into another [19]. Axons of histaminergic neurons spread to all parts of the brain, where they can coordinate the work of other neurotransmitter systems. These neurons play an important role in the regulation of many functions, systems, and reactions of the body: neuroendocrine and cardiovascular, brain blood flow, body temperature, sleep, and wakefulness, eating and drinking behavior, memory, and learning [20]. However, the postnatal development of histaminergic neurons in the rat brain has not been sufficiently studied.
The purpose of this study is evaluation of the Cajal bodies formation features in histaminergic neurons of the rat brain from the 5th to the 45th day of postnatal ontogenesis.
Material and Methods
Animals, Chemicals and Experimental Design
The study was performed on the offspring of outbred white rats (12pups), in accordance with the principles of bioethics and the requirements of the Directive of the European Parliament and of the Council No. 2010/63/EU of September 22, 2010, on the protection of animals used for scientific purposes [21]. The animals were on a standard vivarium diet. Rats that reached the required age were taken out of the experiment by decapitation and the hypothalamus was taken. Decapitation of rat pups was carried out on the 5th, 20th, and 45th days after birth (for a better assessment of the dynamics of development, one rat pup was taken from each litter for each period, a total of 4pups). Identification of brain structures was carried out according to the schemes of the stereotaxic atlas [22]. All the chemicals were obtained from Sigma-Aldrich (USA).
Electron Microscopy
For electron microscopy the lateral parts of posterior hypothalamus, where the histaminergic neurons of the largest group, E2, are situated, immediately after they were taken were placed in 1% osmium fixative in Millonig’s buffer (pH = 7.4) [23] for 2hours at a temperature of +4°C. The samples were washed in a mixture of Millonig’s buffer (20ml) and sucrose (900mg), dehydrated in 50° and 70°ethanol. The samples were kept for 12hours in 70°ethanol. Then they were dehydrated in alcohols of increasing concentration, a mixture of alcohol and acetone, 3 portions of acetone, passed through a mixture of resin (Araldite M + Araldite M hardener 964 + dibutyl phthalate + Araldite M accelerator 960) and acetone, and then they were enclosed in resin in gelatin capsules and placed in a thermostat at a temperature of +60°C for 4days for polymerization. Semithin sections 350nm thick were obtained with a ultramicrotome Leica EM UC 7 (Leica Microsystems GmbH, Germany) and stained with methylene blue. The preparations were examined under a light microscope to clarify the localization of E2 nucleus histaminergic neurons of the hypothalamus. For the identification of the E2 group of histaminergic neurons the stereotaxic atlas and corresponding topographic schemes were used [19,22].
Ultrathin sections (about 35nm thick) were made on a ultramicrotome Leica EM UC 7 (Leica Microsystems GmbH, Germany), collected on supporting copper grids (Sigma, cell size 300×83), and counterstained with uranyl acetate [24] for 20 minutes under a dark cover at room temperature, then washed in three portions of bidi stilled water for 25 seconds, counterstained with lead citrate for 8 minutes [25] and washed in three portions of bidi stilled water for 30 seconds. The resulting preparations were examined with a transmission electron microscope JEM-1011 (JEOL, Japan). Electron micrographs were acquired by digital camera (Olympus Mega View III, Germany). Ultrastructural morphometry was performed using the item image processing program (Version 5.0; Build 1224; Serial Number A3766900-7E852FAB, Germany), tracing selected objects on a computer monitor with the cursor and estimating their number and size.
Statistics
The primary data obtained were treated by nonparametric statistics using software Statistics 10.0 (Stat Soft, Inc., USA). Quantitative results were presented as “Me (LQ; UQ)”, where Me is the median, LQ is the upper limit of the lower quartile, and UQ is the lower limit of the upper quartile. Comparison of groups on one basis was carried out using the Mann-Whitney U-test for independent samples. Differences between groups were considered statistically significant if the probability of an erroneous estimate did not exceed 5% (p<0.05).
Results
It has been established that Cajal bodies, both free in the nucleoplasm and attached to nucleoli, are found in histaminergic neurons nuclei of rats of both sexes throughout the period of postnatal ontogenesis studied here. They exhibit a consistent ultrastructure, even when attached to nucleoli in different stages of histaminergic neurons maturation. In the nuclei of developing histaminergic neurons, the cross-sectional area of Cajal bodies varies from 0.15 to 0.32μm2. They are oval or spherical in shape, not limited by a membrane, and consist of granules, dense spiral filaments, and an intermediate matrix of lower osmiophilic density (Figure 1A).
In 5-day-old rat pups, in the nuclei of half of the histaminergic neurons studied by us, there are from 1 to 3 CBs, predominantly in contact with the nucleoli (78%) (Figure 1B) or lying freely at some distance from them (22%), not revealing topological connections with nuclear envelope (Figure 1A). Their average number in the nucleus is 1.1 (Table 1). In the process of forming a close association with the nucleolus, the CB is attached to it, forming, as a rule, a single cap (“nucleolar cap”) on its surface (Figure 1B). However, a pairwise arrangement of Cajal bodies associated with the same nucleolus was also noted (5% of cases). At the same time, the attachment of CBs occurs more often in the area where the dense fibrillar component of the nucleolus is located (Figure 1) .
On the 20th day of postnatal development in rats, the number of histaminergic neurons, in the nuclei of which Cajal bodies are seen, decreases to 27% (Table 1). At the same time, all detected CBs are associated with the nucleoli (Figure 1C). The average number of CBs is 1.33 (Table 1). Their number, per one and the same nucleolus, increases to an average of 3, and the nature of attachment to it does not change, that is, as before, it is carried out mainly through the dense fibrillar component of the latter. In some cases, up to 5 such structures are found in neurons, which form perinucleolar ring clusters (“rosettes”) when attached to the nucleolus (Figure 2).
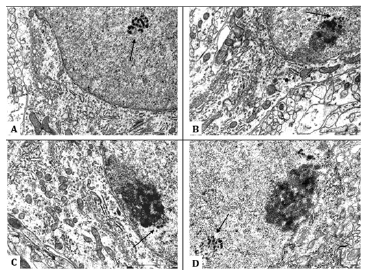
Figure 1:Cajal bodies in the nuclei of developing rat hypothalamus histaminergic neurons. General view of the Cajal body, lying freely in the nucleoplasm, 5th day of postnatal ontogenesis (A). Cajal body associated with the nucleolus, it forms a cap on the surface of the nucleolus, 5th day (B). 20th day (C). of postnatal ontogenesis. Single Cajal body located not far from the nucleolus, 45th day of postnatal ontogenesis (D). Cajal bodies are shown by arrows. Scale bar: 1 μm, ×30000.
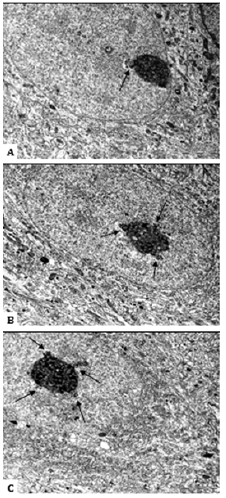
Figure 2:Cajal bodies, attached to nucleoli, in the nuclei of developing rat hypothalamus histaminergic neurons. Single Cajal body associated with the nucleolus (A). Cajal bodies, formed a rosette around the nucleolus, when they associate with it (B, C). 20th day of postnatal ontogenesis. Cajal bodies are shown by arrows. Scale bar: 2 μm, ×12000.
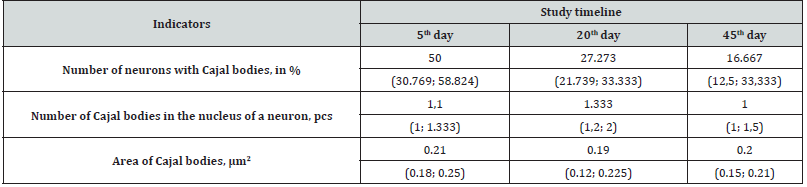
Table 1:Cajal bodies in the nuclei of histaminergic neurons during postnatal development in rats (Me (LQ; UQ)).
By the 45th day of postnatal ontogenesis, the number of histaminergic neurons with Cajal bodies decreases to 17%. At the same time, both nucleolar-associated (70%) and free-lying in the nucleoplasm (30%) CBs are again found (Figure 1D). Their average number is 1.0 (Table 1). The number of Cajal bodies associated with the same nucleolus ranges from 1 to 3. The occurrence frequency of these structures, compared with the 5th day, decreases by almost 2.6 times (Table 1), but their size and appearance do not undergo significant changes (Table 1).
Discussion
During the period of postnatal development in rats, in the nuclei of histaminergic neurons of the hypothalamus, Cajal bodies are found, predominantly in contact with the nucleoli, which, in general, is a common occurrence for rat brain neurons [1]. At the same time, CBs attachment occurs, as a rule, in the area where the dense fibrillar component of the nucleolus is located, which is the site of synthesis and early processing of pre-rRNA [26]. It is known that Cajal bodies share fibrillarin, Nopp140, NAP57, and DNA topoisomerase I proteins with the nucleolus [8,12,17,18]. In the nucleolus, these compounds are in the region of the dense fibrillar component [16], and thus, apparently, serve as the molecular basis for the interaction between these nuclear bodies. This suggests that the frequent associations of CBs with the nucleolus observed in developing histaminergic neurons contribute to the targeted effect of the main components of the pre-rRNA processing mechanism on the nucleolus [2] due to the high cytoplasmic requirements of growing cells for protein biosynthesis.
Interestingly, during the described period of neuronal development, CBs, unlike nucleoli, do not undergo significant changes in shape and size, i.e., they do not show any structural differences that could be associated with the age of animals. The highest frequency of occurrence of these structures is observed on the 5th day. This is generally consistent with data obtained in the study of other types of neurons during their development [27,28].
On the 20th day in the nuclei of histaminergic neurons, the number of Cajal bodies per nucleolus increases to an average of 3, in single cases up to 5 such formations are found in cells, forming rosettes around the nucleoli. It is assumed that nuclei with a large number of CBs support a higher assembly rate of small nuclear and nucleolar ribonucleoproteins due to an increased likelihood of molecular interactions between CBs components surrounding the nucleolus [29,30]. It is known that growing neurons have high transcriptional activity [31-33]. Since the accessory bodies of Cajal are transcriptionally dependent nuclear structures [2,16] and are most common in rapidly developing cells [17,27,34], the presence of these bodies clusters in the form of rosettes around the nucleolus, which we observed at 20th day of postnatal development of histaminergic neurons is quite natural. Their number dynamically adapts to maintain the high demand of neurons for splicing and biogenesis of ribosomes, which are necessary to stabilize metabolic and bioelectrical activity [16].
Similar peri nucleolar clusters of Cajal bodies have been noted previously when studying the development of pyramidal neurons in the hippocampus of rats after 13-14th days of postnatal development at the stage of their terminal differentiation [35]. Similar rosettes were also observed in neurons in other areas of the brain, and therefore, it was suggested that this phenomenon might be a kind of marker for the final stage of neuronal differentiation.
Thus, in the postnatal ontogenesis of histaminergic neurons, the connection between Cajal bodies and the nucleolus can be characterized as supportive, given that CBs promote the formation of mature components in the nucleoli that are necessary for ribosome biogenesis.
Conclusion
From the 5th to the 45th day of postnatal ontogenesis, in the nuclei of histaminergic neurons of the hypothalamus of rats, Cajal bodies are predominantly in contact with the nucleolus or lying freely. In the process of forming a close association with the nucleolus, Cajal body is attached to it, forming a “cap” on its surface. On the 20th day, an increase in the degree of association of the nucleoli with Cajal bodies is revealed, which is manifested by the formation of peri nucleolar ring clusters from Cajal bodies in the form of rosettes. During the described period of postnatal ontogenesis of histaminergic neurons, Cajal bodies do not undergo significant changes in shape and size, which could be associated with the age of animals. The occurrence frequency of these structures decreases by almost 2.6 times.
Author Contributions
All authors made the same contribution to this study and the preparation of the article.
Conformity with the Principles of Ethics
The study was performed in accordance with the principles of bioethics and the requirements of the Directive of the European Parliament and of the Council No. 2010/63/EU of September 22, 2010, on the protection of animals used for scientific purposes.
Acknowledgements
The authors are grateful to the team of the Morphology and Electron Microscopy Group of the Research Laboratory of the Grodno State Medical University for their assistance in the research.
Conflict of Interest
Authors declare that they have no financial or personal conflicts of interest that could inappropriately influence the conduct of this research.
References
- Raska I, Ochs RL, Andrade LE, Chan EK, Burlingame R, et al (1990) Association between the nucleolus and the coiled body. J Struct Biol 104(1-3): 120-127.
- Pena E, Berciano MT, Fernandez R, Ojeda JL, Lafarga M (2001) Neuronal body size correlates with the number of nucleoli and Cajal bodies, and with the organization of the splicing machinery in rat trigeminal ganglion neurons. J Comp Neurol 430(2): 250-263.
- Monneron A, Bernhard W (1969) Fine structural organization of the interphase nucleus in some mammalian cells. J Ultrastruct Res 27(3): 266-288.
- Hardin JH, Spicer SS, Greene WB (1969) The paranucleolar structure, accessory body of Cajal, sex chromatin, and related structures in nuclei of rat trigeminal neurons: a cytochemical and ultrastructural study. Anat Rec 164 (4): 403-431.
- Lafarga M, Hervás JP, Santa-Cruz MC, Villegas J, Crespo D (1983) The “accessory body” of Cajal in the neuronal nucleus. A light and electron microscopic approach. Anat Embryol (Berl) 166(1): 19-30.
- Carvalho T, Almeida F, Calapez A, Lafarga M, Berciano MT, et al (1999) The spinal muscular atrophy disease gene product, SMN: a link between snRNP biogenesis and the Cajal (coiled) body. J Cell Biol 147(4): 715-728.
- Gall JG, Bellini M, Wu Z, Murphy C (1999) Assembly of the nuclear transcription and processing machinery: Cajal bodies (coiled bodies) and transcriptosomes. Mol Biol Cell 10(12): 4385-4402.
- Gall JG (2000) Cajal bodies: the first 100 years. Annu Rev Cell Dev Biol 16(1): 273-300.
- Mao YS, Zhang B, Spector DL (2011) Biogenesis and function of nuclear bodies. Trends Genet 27(8): 295-306.
- Gilder A, Hebert M (2011) Chapter 16: Relationship of the Cajal body to the nucleolus. In: Olson MOJ, The nucleolus (Protein Reviews, Vol. 15). pp.361-380.
- Trinkle-Mulcahy L, Sleeman JE (2017) The Cajal body and the nucleolus: "In a relationship" or "It's complicated"? RNA Biol 14(6): 739-751.
- Matera AG (1999) Nuclear bodies: multifaceted subdomains of interchromatin space. Trends Cell Biol 9(8): 302-309.
- Machyna M, Heyn P, Neugebauer KM (2013) Cajal bodies: where form meets function. Wiley Interdiscip Rev RNA 4(1):17-34.
- Stanek D, Neugebauer KM (2006) The Cajal body: a meeting place for spliceosomal snRNPs in the nuclear maze. Chromosoma 115(5): 343-354.
- Novotny I, Malinova A, Stejskalova E, Mateju D, Klimesova K, et al (2015) SART3-Dependent Accumulation of incomplete spliceosomal snRNPs in Cajal bodies. Cell Rep 10(3): 429-440.
- Lafarga M, Tapia O, Romero AM, Berciano MT (2017) Cajal bodies in neurons. RNA Biol 14(6): 712-725.
- Lamond AI, Carmo-Fonseca M (1993) The coiled body. Trends Cell Biol 3(6): 198-204.
- Isaac C, Yang Y, Meier UT (1998) Nopp 140 functions as a molecular link between the nucleolus and the coiled bodies. J Cell Biol 142(2): 319-329.
- Zimatkin SM, Kuznetsova VB, Strik ON (2006) Spatial organization and morphometric characteristics of histaminergic neurons in the rat brain. Neurosci Behav Physiol 36(5): 467-471.
- Haas H, Sergeeva O, Selbach O (2008) Histamine in the nervous system. Physiol Rev 88(3): 1183-1241.
- (2010) Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes: text with EEA relevance. Official J European Union.
- Paxinos G, Watson C (2007) The Rat Brain in Stereotaxic Coordinates. (6th). London: Academic Press.
- Millonig G (1961) Аdvanvantges of a phosphate buffer for OsO4 solutions in fixation. J Appl Physics 32: 1637-1643.
- Watson ML (1958) Staining of tissue sections for electron microscopy with heavy metals. J Biophys Biochem Cyt 4(4): 475-478.
- Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17(1): 208-212.
- Boisvert FM, van Koningsbruggen S, Navascues J, Lamond AI (2007) The multifunctional nucleolus. Nat Rev Mol Cell Biol 8 (7): 574-585.
- Hervás JP, Villegas J, Crespo D, Lafarga M (1980) Coiled bodies in supraoptic nuclei of the rat hypothalamus during the postnatal period. Am J Anat 159(4): 447-454.
- Clark P, Jones KJ, LaVelle A (1990) Ultrastructural and morphometric analysis of nucleolar and nuclear changes during the early growth period in hamster facial neurons. J Comp Neurol 302(4): 749-760.
- Klingauf M, Stanek D, Neugebauer KM (2006) Enhancement of U4/U6 small nuclear ribonucleoprotein particle association in Cajal bodies predicted by mathematical modeling. Mol Biol Cell 17(12): 4972-4981.
- Strzelecka M, Trowitzsch S, Weber G, Luhrmann R, Oates AC, et al (2010) Coilin-dependent snRNP assembly is essential for zebrafish embryogenesis. Nat Struct Mol Biol 17(4): 403-409.
- Hernandez-Verdun D (2011) Chapter 1: Structural organization of the nucleolus because of the dynamics of ribosome biogenesis. In: Olson MOJ, The nucleolus (Protein Reviews, Vol. 15). pp.3-28.
- Lafarga M, Villegas J, Crespo D (1985) Changes in nucleolar morphology and volume of the supraoptic nucleus neurons during postnatal development of the rat. Brain Res 354(2): 310-313.
- Zimatkin SM, Zaerko AV, Fedina EM (2021) Nucleoli in developing histaminergic neurons in the rat brain. Neuroscience and Behavioral Physiology 51(4): 535-540.
- Spector DL (1993) Macromolecular domains within the cell nucleus. Annu Rev Cell Biol 9: 265-315.
- Santama N, Dotti CG, Lamond AI (1996) Neuronal differentiation in the rat hippocampus involves a stage-specific reorganization of subnuclear structure both in vivo and in vitro. Eur J Neurosci 8(5): 892-905.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.